Abstract
Introduction:-
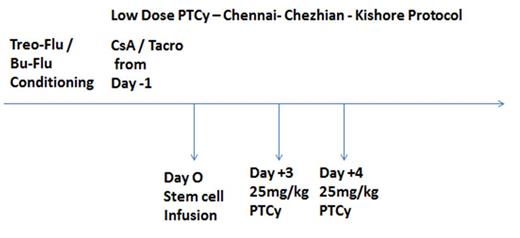
Haploidentical Bone marrow transplantation has become one of the most commonly employed alternative donor techniques, with many centers applying T-cell-replete strategies developed by the Baltimore group using high-dose post-transplant cyclophosphamide. The usefulness of this technique is to reduce graft acquisition cost, lesser grade of GVHD, and reduction in non-relapse mortality. One problem we face in a country like India is the worry of engraftment delay leading to refractory multi-drug resistant gram-negative sepsis increasing the risk of transplant-related mortality. We took up this pilot study to check for the feasibility of a low dose (50%) 25mg/kg of cyclophosphamide as PTCy and its impact on transplant outcomes especially GVHD.
Methodology:-
This study was done from January 2016 to June 2019. 41 Haploidentical bone marrow transplants were done for Acute Myeloid leukemia at this time under this protocol. 23 Cases had Busulfan / Fludarabine as conditioning and in 18 cases; we used Treosulfan / Fludarabine-based conditioning regimen. The study protocol PTCy dose was 25mg/kg on Day +3 and Day +4. Cyclosporine / Tacrolimus was used from day -1 in all patients as GVHD prophylaxis. The parameters analyzed are engraftment dynamics, rates of gram-negative sepsis, hemorrhagic cystitis, rates of acute and chronic GVHD, and 2-year Non-relapse mortality.
Results:
Parents were the haploidentical donors in 22 cases whereas siblings in the rest 19 cases. Peripheral blood stem cell collection was chosen in all cases. The average CD34 dose was 6.8 x 10 6/Kg of the recipient. The mean number of days to achieve engraftment was around day 11. Culture-proven gram-negative sepsis was seen in 11 cases (26.8%). Two patients died within Day +30. Both due to sepsis. The rates of acute GVHD of grade I/2 was 18 (43.9%) and grade 3/4 acute GVHD was seen only in 2 patients (5%). No mortality was due to GVHD. With a median follow-up of two years, chronic skin and liver grade 1/2 GVHD was noted in 29 patients (74.3%) but were manageable with oral and topical medications not needing admission. Three patients had hemorrhagic cystitis. CMV reactivation needing medications was seen in 5(12.2%) patients indicating a higher incidence of post-transplant viral reactivation with PTCy. At data cut-off, we had lost 12 patients due to relapse and there was no non-relapse mortality except for the two sepsis-related death.
Conclusion:
The results of this pilot study indicate the feasibility of using a low dose PTCy (25mg/kg) in Haploidentical transplant as a methodology for in vivo T cell depletion without increased incidence of GVHD and Non-relapse mortality in Indian patients. This is a small study and it needs further follow-up with drug metabolism analysis and a bigger cohort to make this Chennai-Chezhian-Kishore PTCy Protocol a validated standard in Haplo-identical transplants. This is a pilot study and we are planning to incorporate genetic polymorphism in drug metabolism to validate the proposed protocol more scientifically.
Reference:
1. Wang, Y et al. Low-dose post-transplant cyclophosphamide and anti-thymocyte globulin as an effective strategy for GVHD prevention in haploidentical patients. J Hematol Oncol 12, 88 (2019). https://doi.org/10.1186/s13045-019-0781-y
2. Shannon R. McCurdy, Leo Luznik How we perform haploidentical stem cell transplantation with posttransplant cyclophosphamide Blood (2019) 134 (21): 1802-1810.
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal